Stress
The brain is the primary control center for our entire body and can be affected by stress in many ways. The stress-inducing stimuli are referred to as stressors. The physiological and behavioral changes in response to exposure to stressors represent the stress response. All physical or psychological stressors that disturb the natural homeostasis (dynamic balance) lead to a stress reaction. A stress reaction is mediated by a complex interplay of nervous, endocrine and immunological mechanisms. The stress response itself is an important function of the body. It helps us to prepare for dangers or react to emergencies [1].
The physiology of the stress response
The stress response takes place via 2 stress axes: the faster neuronal axis via the sympathetic nerve cord (sympathetic-adrenal medullary axis, abbreviated SNA or SAM) and the somewhat slower hormonal axis (hypothalamic-pituitary-adrenal axis, abbreviated HHNA or HPA axis).
Faster stress response via the sympathetic-adrenal axis
The faster stress response via the Sympathetic-adrenal medullary axis (SNA or SAM) causes rapid reactions of the target organs via adrenergic and noradrenergic neurons (neurons that are activated by the neurotransmitters adrenaline and noradrenaline) and mediates motor activity. The sympathetic nervous system (part of the autonomic nervous system responsible for performance and activity) activates the adrenal medulla, which releases adrenaline and noradrenaline (catecholamines). These processes reduce the work of digestion, blood pressure, blood flow of the skeletal muscles, blood sugar level and the heart rate are increased and the breathing is Bronchodilatation facilitated. This increases the oxygen and glucose supply and there is increased vigilance, increased brain performance and pain relief. The organism is now in an optimal flight or fight readiness (flight or fight response) [2].
Slower stress response via the hypothalamic-pituitary-adrenal axis
The slower stress response via the hypothalamic-pituitary-adrenal axis (HHNA or HPA axis) puts the body into a somewhat delayed ‘state of emergency’ and mediates stress adaptations in areas of the metabolism and immune system via various hormones, such as cortisol. The most important effects of cortisol include Increase in blood sugar as well as protein and fat breakdown, inhibition of protein synthesis and tissue formation (e.g. in skin), collagen, blood vessels and bones (long-term: osteoporosis), inhibition of immune cells and inflammatory mediators such as interleukins, interferon or histamine, reduced secretion of sex hormones and other hormones. It also causes inhibition of memory, information processing, sexuality, sleep as well as inhibition of neuronal pathways, networking and differentiation. In this way, long-term release of cortisol has an inhibitory and destabilizing effect on the CNS and is significantly involved in the pathophysiological developments associated with long-term stress [2].
The stress response is initially adaptive, i.e. it prepares the body to cope with the challenges posed by the stressor. For example, the body’s physiological reactions to trauma and an invasive procedure serve to mitigate further tissue damage. However, if the exposure to a stressor is actually or perceived to be intense, repeated (repeated acute stress) or continuous over a longer period of time (chronic stress), the stress response becomes maladaptive and damages the physiology. In this way, the burden of chronic stressors and constant cortisol release can be reduced via the hypothalamic-pituitary-adrenal axis can cause maladaptive responses such as depression, anxiety, cognitive impairment and heart disease [3].
Link between chronic stress and Alzheimer’s disease
Chronic stress is associated with an increased risk of Alzheimer’s disease. A long-term study conducted by the Swedish University of Gothenburg in 2013 showed that the risk of developing dementia correlated directly with the number of stressful events experienced. Even past stressful experiences can contribute to increased stress levels even after many years [4]. Furthermore, a recent cohort study involving 1,362,548 people between the ages of 18 and 65 in the Stockholm region found that chronic stress increases the risk of mild cognitive impairment and Alzheimer’s disease by 2.45 times [5]. In addition, studies using imaging techniques (magnetic resonance imaging) found that hippocampal regions were 5 to 26% smaller in patients under stress compared to healthy subjects [6]. In these studies, the severity of the trauma experienced by the patients correlated with the degree of brain shrinkage. As the hippocampus is the site of memory consolidation and is the first to be destroyed in Alzheimer’s disease, these studies clearly demonstrate the link between chronic stress and the acceleration of the development of Alzheimer’s disease.
The vicious circle of stress
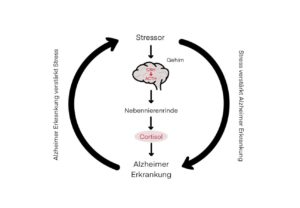
Fig 1: The vicious circle of stress, proposed by Justice (2018)
The molecular biologist Nicholas Justice illustrated the extremely complex relationship between stress and Alzheimer’s disease with a highly simplified construct, the “vicious circle of stress” [7].
In the “vicious circle of stress”, the right-hand side of the cycle stands for the negative influence of stress on neurodegenerative diseases such as Alzheimer’s disease. According to this study, chronic stress and the associated elevated cortisol levels exacerbate Alzheimer’s disease by permanently activating the hypothalamic-pituitary-adrenal axis (center) and lead to a more rapid development of neuronal pathology and loss of cognitive functions, which has already been experimentally proven in numerous studies [7]. However, there are far fewer studies that have looked at the left-hand side of the vicious circle. Here it is shown that Alzheimer’s disease disrupts the neuronal and endocrine circuits of the stress response, which in turn leads to neuropsychiatric comorbidities such as depression, anxiety, insomnia and aggressive behavior. This in turn can accelerate the progression of Alzheimer’s disease and lead to neuropsychiatric complications [7]. According to this, stress and Alzheimer’s disease would reinforce each other.
Stress is measurable
Chronic stress can also be measured objectively. Physiological measurements such as the analysis of heart rate variability (HRV), the measurement of cortisol levels and catecholamines (adrenaline and noradrenaline) can be used to objectively measure.
HRV is a measure of the temporal fluctuations between the individual heartbeats, which are controlled by the autonomic nervous system. It reflects the body’s ability to adapt to change and can be an indicator of general well-being, stress levels and fitness readiness. A high HRV is associated with higher cardiovascular fitness and stress resistance. However, HRV decreases with increasing age, while norepinephrine levels in the blood rise at the same time. These changes lead to a reduced ability to adapt to stress and can increase the risk of various health problems such as cardiovascular disease, depression, anxiety and Alzheimer’s disease.
Stress management through cardiac coherence breathing leads to a reduction in biomarkers of Alzheimer’s disease
One glimmer of hope is a groundbreaking study suggesting that a special breathing technique could reduce stress and possibly lower the risk of Alzheimer’s disease.
Breathing helps to regulate your own heart rhythm and can lead to a state of heart rhythm coherence (or heart coherence for short). Cardiac coherence describes the state in which the respiratory rate and heart rate are in harmony. Cardiac coherence breathing, also known as resonance breathing, therefore corresponds to the breathing frequency at which the heart rate and breathing are most closely matched. During coherent breathing, the heart rate variability HRV (variation in the time intervals between heartbeats) is at its highest. To achieve heart coherence, you need to consciously slow down your breathing using a specific technique, breathing slowly and focusing on your heart, while suggesting positive emotions such as serenity, gratitude, appreciation or compassion. A number of important physiological changes occur during coherence. The two branches of the autonomic nervous system synchronize with each other and there is a general shift towards increased parasympathetic activity (parasympathetic = recreational part of the autonomic nervous system).
The study used emWave® Pro software developed by HeartMath to train participants in slow cardiac coherence breathing. The participants were divided into two groups: The intervention group practiced slow coherence breathing at a frequency of 0.1 HZ with the HeartMath system, which demonstrably brought the participants into the heart coherence frequency. The software-controlled HRV biofeedback in real time enabled the participants to optimize their breathing technique. The control group, on the other hand, was instructed to use individual strategies to increase their HRV. The breathing techniques were performed for 20-40 minutes a day in both groups. In order to record effects on brain physiology, various biomarkers of Alzheimer’s disease were measured in both groups.
The results were astounding. The research team was able to show that just four to five weeks of slow, heart-coherent breathing using HRV biofeedback had a positive effect on the following biomarkers of Alzheimer’s disease:
- The hippocampus volume increased in older adults [4].
- Plasma levels of Alzheimer’s-specific proteins (amyloid-ß) were reduced in both younger and older adults (possibly by reducing the production of amyloid-ß and increasing the cellular disposal of amyloid-ß) [5].
- The cortical volume (volume of the cerebral cortex) and coordination as well as emotion regulation increased in younger and older adults [6].
What is impressive about this study is that these changes in brain structure, function and health were achieved through simple daily exercise. If such effects had been achieved by a pharmaceutical company, they would undoubtedly be advertised in all the world’s media. And in contrast to the latest pharmaceutical antibody candidates which have so far shown little patient benefit and a high health risk due to side effects, there were no undesirable side effects with this respiratory intervention.
You too can bring your heart into coherence through targeted breathing with HRV biofeedback, as the HeartMath system tested in the study is commercially available. With just a few minutes of breathing training a day, you can simply “breathe away” stress and make a positive contribution to your (brain) health!
References
- Mifsud, K. R., & Reul, J. M. H. M. (2018). Mineralocorticoid and glucocorticoid receptor-mediated control of genomic responses to stress in the brain. Stress (Amsterdam, Netherlands), 21(5), 389-402. https://doi.org/10.1080/10253890.2018.1456526
- Chu, B., Marwaha, K., Sanvictores, T., & Ayers, D. (2021). Physiology, stress reaction. InStatPearls [Internet]. StatPearls Publishing. https://www.researchgate.net/publication/335925700_Physiology_Stress_Reaction
- Ketchesin, K. D., Stinnett, G. S., & Seasholtz, A. F. (2017). Corticotropin-releasing hormone-binding protein and stress: from invertebrates to humans. Stress (Amsterdam, Netherlands), 20(5), 449-464. https://doi.org/10.1080/10253890.2017.1322575
- Johansson, L., Guo, X., Hällström, T., Norton, M. C., Waern, M., Ostling, S., Bengtsson, C., & Skoog, I. (2013). Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer’s disease: a 38-year longitudinal population study. BMJ open, 3(9), e003142. https://doi.org/10.1136/bmjopen-2013-003142
- Wallensten, J., Ljunggren, G., Nager, A. et al. Stress, depression, and risk of dementia – a cohort study in the total population between 18 and 65 years old in Region Stockholm. Alz Res Therapy 15, 161 (2023). https://doi.org/10.1186/s13195-023-01308-4
- Bering, R., Eisbo, C., Fischer, G., & Johansen, F. F. (2005). Neurovulnerability of the hippocampal formation in post-traumatic stress disorder. PPmP-Psychotherapie- Psychosomatik- Medizinische Psychologie, 55(02). https://www.researchgate.net/publication/273033010_Neurovulnerabilitat_der_Hippokampusformation_bei_der_Posttraumatischen_Belastungsstorung
- Justice N. J. (2018). The relationship between stress and Alzheimer’s disease. Neurobiology of stress, 8, 127-133. https://doi.org/10.1016/j.ynstr.2018.04.002
- Yoo, H. J., Nashiro, K., Dutt, S., Min, J., Cho, C., Thayer, J. F., Lehrer, P., Chang, C., & Mather, M. (2023). Daily biofeedback to modulate heart rate oscillations affects structural volume in hippocampal subregions targeted by the locus coeruleus in older adults but not younger adults. medRxiv : the preprint server for health sciences, 2023.03.02.23286715. https://doi.org/10.1101/2023.03.02.23286715
- Min, J., Rouanet, J., Martini, A.C. et al. Modulating heart rate oscillation affects plasma amyloid beta and tau levels in younger and older adults. Sci Rep 13, 3967 (2023). https://doi.org/10.1038/s41598-023-30167-0
- Yoo, H. J., Nashiro, K., Min, J., Cho, C., Bachman, S. L., Nasseri, P., Porat, S., Dutt, S., Grigoryan, V., Choi, P., Thayer, J. F., Lehrer, P. M., Chang, C., & Mather, M. (2022). Heart rate variability (HRV) changes and cortical volume changes in a randomized trial of five weeks of daily HRV biofeedback in younger and older adults. International journal of psychophysiology : official journal of the International Organization of Psychophysiology, 181, 50-63. https://doi.org/10.1016/j.ijpsycho.2022.08.006



