Gluten, also known as gluten protein, owes its name to its two main components: gliadin and glutenin. Gliadine sind große Proteinmoleküle, die reich an den Aminosäuren Glutamin und Prolin sind. Sie können wiederum in Subgruppen unterteilt werden, alpha-Gliadine, beta-Gliadine, Gamma-Gliadine und Omega-Gliadine. Von Letzteren wird insbesondere das Omega-5-Gliadin mit Nahrungsmittelunverträglichkeiten in Verbindung gebracht.
Gliadins ensure the elasticity and binding of the dough during production. Glutenins, on the other hand, are responsible for elasticity and viscosity [19]. Together they make up around 80-85% of the total protein in wheat.
The history of wheat goes back to the beginnings of agriculture. Original varieties such as einkorn and emmer were developed through breeding into higher-yielding, more disease-resistant and more bakeable varieties. However, different types of wheat (einkorn, emmer, spelt, durum and soft wheat) show large differences in the composition of their proteins. Wheat (common and durum wheat) contains the highest amounts of potentially allergenic proteins, including omega-5 gliadin. Spelt and emmer have slightly reduced amounts, about half as much as common wheat. Einkorn contains the lowest amounts, with a reduction of around 5.4 times that of common wheat [1].
This could explain why the increased consumption of wheat products has led to an increase in gluten-related diseases. A meta-analysis shows that the number of coeliac disease diagnoses has risen by around 7.5% annually in recent decades [5]. In Germany, around 1% of the population – i.e. one in 100 people – is affected. Recent research findings indicate that gluten-related diseases, in particular coeliac disease, not only affect the gut but can also cause neurological symptoms. These include brain fog, memory loss, disorientation and confusion. It is striking that these symptoms are similar to those of neurodegenerative diseases such as Alzheimer’s. There is evidence that celiac disease and gluten sensitivity may be indirectly related to various forms of dementia such as Alzheimer’s disease, frontotemporal dementia and vascular dementia [8,10,11]. These symptoms may occur due to communication between the gut and the brain.
Communication between the gut and the brain
Communication between the gut and brain is crucial for the exchange of chemical and physical substances, which ensures the smooth functioning of both organs. This exchange takes place via various systems, such as the gut-brain axis and via the bloodstream through the blood-brain barrier.
The gut-brain axis
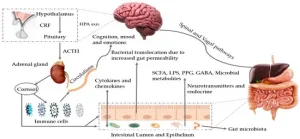
Image 2
The gut-brain axis is a highly complex communication system that facilitates the exchange between the gut and the brain. Around 90 % of the signals flow from the gut to the brain, only 10 % in the opposite direction. This exchange takes place via nerve connections, immune reactions as well as messenger substances and metabolic products produced by the intestinal microbiome [2,11,16,17].
The blood-brain barrier
Substances can be transported from the intestine to the brain via the bloodstream. The blood-brain barrier is a protective barrier that protects the brain from harmful substances in the blood. It ensures that only important nutrients and waste products reach the brain. At the same time, it prevents large molecules and toxic substances from entering the brain [12].
How does gluten lead to dementia?
Although the exact mechanisms are not yet known, there are some theories that are still in the early stages of research.
Theory 1: Leaky-good leads to leaky-brain
Gluten ingested with food reaches the small intestine via the mouth and stomach. In the stomach and small intestine, digestive enzymes break down the gluten into glutenin and gliadin, whereby gliadin cannot be completely broken down into its individual amino acids. The undigested gliadin reaches the intestine, where it can trigger so-called leaky gut, particularly in gluten-sensitive people. This clinical picture is characterized by damage to the intestinal wall, which can lead to the destruction of its barrier function. This can trigger a permanent activation of the intestine-associated immune system and thus contribute to chronic inflammation. This can spread to the central nervous system and thus to the brain. The intestinal colonization, the intestinal microbiome, can also be adversely affected by gluten. Both mechanisms are described in detail below.
Destruction of the intestinal wall
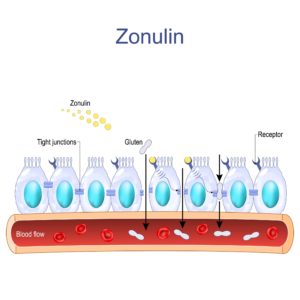
Image 3
Udigested gluten proteins, especially gliadin, bind to the intestinal cells (epithelial cells) that form the intestinal wall via various receptors. In particular, binding to the receptor CXCR3 triggers a signaling cascade that leads to increased production and release of a protein called zonulin. Zonulin then binds to special receptors on the epithelial cells, which loosens the junctions between the epithelial cells, the so-called tight junctions. This literally creates “gaps” between the epithelial cells, making the intestinal wall more permeable [3,4,7].
Through a permeable intestinal barrier, undigested food components such as gliadins, toxic metabolic products and surface proteins of intestinal bacteria such as LPS (lipopolysaccharides), microorganisms or cytokines can enter the bloodstream directly. The penetration of these substances activates the immune system and leads to the release of pro-inflammatory cytokines such as IL-6, IL-1β and TNF-α. Via the bloodstream, these cytokines finally reach the blood-brain barrier, which also becomes more permeable as a result of these inflammatory processes, which can lead to the so-called “leaky brain”.
Due to the “leaky gut”, undigested food components, pathogens and metabolic products of the microbiome can increasingly enter the bloodstream. Through the “leaky brain”, these substances also increasingly enter the brain. There they lead to increased neuroinflammation and oxidative stress or, in the case of LPS (lipopolysaccharides), even to the increased formation of plaques, which increases the risk of neurological diseases such as dementia [12].
Changes in the gut microbiome
In people with coeliac disease, the gut microbiome (all bacteria in the gut) can be altered by a gluten-containing diet or gluten-induced inflammation, resulting in an imbalance between the physiological and pathogenic bacteria in the gut (dysbiosis) [12,15].
Our physiological gut bacteria produce important metabolic products such as short-chain fatty acids (SCFA) or neurotransmitters (e.g. the happiness hormone serotonin or GABA). An imbalance in our microbiome in favor of pathogenic germs can lead to increased production of lipopolysaccharides (LPS), which can trigger inflammation and thus also impair the blood-brain barrier. Toxic metabolic products of these pathogenic bacteria can also enter the bloodstream via the intestine and potentially also the brain. In addition, dysbiosis leads to a reduced number of acid-forming flora and thus to a reduced synthesis of short-chain fatty acids (SCFAs), which have an anti-inflammatory effect [17]. Furthermore, the production of neurotransmitters such as GABA and serotonin can also be impaired. Reduced production of these neurotransmitters can have a negative impact on cognitive function and mood regulation [12].
An altered gut microbiome and changes in metabolic products have also been demonstrated in Alzheimer’s patients [6,17]. These findings come mainly from animal and in vitro studies, and more research is needed to better understand the exact relationships.
Theory 2: molecular mimicry and cross-reactivity
In patients suffering from coeliac disease or gluten sensitivity, the immune system falsely recognizes gluten peptides such as gliadin as harmful. In response, the immune system in the intestine, which acts as an essential immunological barrier for the body, forms special antibodies against the gluten proteins. Studies show that gluten proteins have similarities in their amino acid sequence to certain proteins in the brain. This similarity can lead to the gluten antibodies also attacking brain proteins – this is known as cross-reactivity. Such attacks can lead to inflammatory reactions or functional disorders in the brain.
However, the mechanism of these cross-reactions has not yet been fully proven and remains largely hypothetical. Further research is needed to better understand the exact relationships and effects. The following brain proteins are thought to be affected by this cross-reactivity:
- Transglutaminase 6 (TG6): TG6 is an enzyme that is found in certain nerve cells, particularly in the cerebral cortex and cerebellum. It helps with the cross-linking of proteins, supports the development of nerve cells and regulates the production of messenger substances such as GABA, which are necessary for communication between nerve cells. If TG6 is attacked by antibodies, this leads to inflammation of the nerve cells and nerve damage. Such antibodies often occur in people with gluten-related neurological diseases such as gluten ataxia or gluten-related nerve damage. Impaired function of TG6 is also associated with Alzheimer’s disease [14,18].
- Synapsin I: The protein synapsin I is found in many nerve endings in the brain and coats synaptic vesicles. There it regulates, for example, the release of messenger substances (neurotransmitters) and influences other functions at the presynaptic level. Alteration of synapsin I, or its destruction by antibodies, would impair neurotransmitter release, synaptic function and neuronal communication and is associated with Alzheimer’s disease [9,18].
- Alpha-synuclein: Alpha-synuclein is a protein that is found in nerve cells. There it controls the activity at the connection points between the nerve cells (synapses) and regulates the release of messenger substances (neurotransmitters). Under certain pathological conditions, for example when attacked by antibodies, alpha-synuclein can aggregate and clump together. These clumps are called Lewy bodies. They accumulate in the nerve cells and can lead to their death. The formation of Lewy bodies is directly linked to so-called Lewy body dementia [9].
- Amyloid beta precursor protein (APP) and amyloid beta A4: APP is an important protein in the brain that supports the development and function of nerve cells. When APP is broken down into smaller fragments, amyloid beta (Aβ) is formed, among other things. In Alzheimer’s patients, this Aβ clumps together to form so-called amyloid plaques, which are deposited between the nerve cells and disrupt their function. If antibodies cross-react with APP, this can impair the normal function and processing of APP. This could either result in more Aβ or disrupt the degradation of Aβ. These changes increase the risk of the formation of harmful amyloid plaques, which play a role particularly in Alzheimer’s disease in older age (after the age of 65) [9].
- Microtubule-Associated Protein Tau: The tau protein is important for the stability of microtubules in nerve cells. Microtubules are like tiny rails that help the cells to transport nutrients and signals. In Alzheimer’s disease, the tau proteins clump together, leading to microtubule instability and nerve cell death. Cross-reactivity with antibodies with these structures could favor this mechanism [9].
- Cerebellar Degeneration-Related Antigen 1 (CDR1): The CDR1 protein is mainly found in the cerebellum and plays a central role in the control and coordination of movements. If CDR1 proteins are attacked by antibodies, this can lead to the breakdown of cells in the cerebellum. This can lead to movement coordination disorders, which is also symptomatically observed in Alzheimer’s patients [9].
Conclusion
There are indications of a possible link between gluten in people with coeliac disease and gluten sensitivity and an increased risk of dementia. There are various hypotheses and initial research that primarily link a barrier disorder of the intestine (leaky gut syndrome) and the cross-reactivity of antibodies with certain brain proteins to neurodegenerative symptoms and neurological diseases such as dementia. For the general population who do not suffer from coeliac disease and gluten sensitivity, there is currently no clear evidence of a link between gluten consumption and an increased risk of dementia. Many of the findings are based on i n vitro– and animal studies. Further controlled studies in humans are required to confirm a causal relationship.
If you suffer from coeliac disease, gluten sensitivity or a wheat allergy, it is very important to avoid gluten completely or partially. Get professional advice on this.
For healthy people, however, gluten is unproblematic in normal quantities and can be part of a balanced diet. Nevertheless, it should be noted that in the Western diet, foods such as bread, pasta and pastries – and therefore products containing gluten – are often consumed in large quantities. It also depends on the type of wheat you choose: While wheat in particular poses a greater allergenic risk due to the higher proportion of particularly inflammatory Since wheat has omega-5 gliadin, more original wheat varieties such as einkorn, emmer and spelt seem to be more recommendable alternatives. In any case, it is also advisable to add variety to your diet by including other sources of carbohydrates such as pulses, potatoes or wholegrain alternatives to ensure a balanced diet. ensure a balanced diet.
If you would like to find out more about gluten-related diseases or a gluten-free diet, click here.
References
- Afzal, M., Sielaff, M., Distler, U., Schuppan, D., Tenzer, S., & Longin, C. F. H. (2023). Reference proteomes of five wheat species as starting point for future design of cultivars with lower allergenic potential. NPJ Science of Food, 7(1), 9. https://doi.org/10.1038/s41538-023-00188-0
- Carabotti, M., Scirocco, A., Maselli, M. A., & Severi, C. (2015). The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology : Quarterly Publication of the Hellenic Society of Gastroenterology, 28(2), 203. https://pmc.ncbi.nlm.nih.gov/articles/PMC4367209/
- Fasano, A. (2020). All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research, 9, F1000 Faculty Rev-69. https://doi.org/10.12688/f1000research.20510.1
- Hollon, J., Leonard Puppa, E., Greenwald, B., Goldberg, E., Guerrerio, A., & Fasano, A. (2015). Effect of Gliadin on Permeability of Intestinal Biopsy Explants from Celiac Disease Patients and Patients with Non-Celiac Gluten Sensitivity. Nutrients, 7(3), 1565-1576. https://doi.org/10.3390/nu7031565
- King, J. A., Jeong, J., Underwood, F. E., Quan, J., Panaccione, N., Windsor, J. W., Coward, S., deBruyn, J., Ronksley, P. E., Shaheen, A.-A., Quan, H., Godley, J., Veldhuyzen van Zanten, S., Lebwohl, B., Ng, S. C., Ludvigsson, J. F., & Kaplan, G. G. (2020). Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Official Journal of the American College of Gastroenterology | ACG, 115(4), 507. https://doi.org/10.14309/ajg.0000000000000523
- Kowalski, K., & Mulak, A. (2019). Brain-Gut-Microbiota Axis in Alzheimer’s Disease. Journal of Neurogastroenterology and Motility, 25(1), 48-60. https://doi.org/10.5056/jnm18087
- Lammers, K. M., Lu, R., Brownley, J., Lu, B., Gerard, C., Thomas, K., Rallabhandi, P., Shea-Donohue, T., Tamiz, A., Alkan, S., Netzel-Arnett, S., Antalis, T., Vogel, S. N., & Fasano, A. (2008). Gliadin Induces an Increase in Intestinal Permeability and Zonulin Release by Binding to the Chemokine Receptor CXCR3. Gastroenterology, 135(1), 194-204.e3. https://doi.org/10.1053/j.gastro.2008.03.023
- Lanza, G., Bella, R., Cantone, M., Pennisi, G., Ferri, R., & Pennisi, M. (2018). Cognitive Impairment and Celiac Disease: Is Transcranial Magnetic Stimulation a Trait d’Union between Gut and Brain? International Journal of Molecular Sciences, 19(8), Article 8. https://doi.org/10.3390/ijms19082243
- Lerner, A., & Benzvi, C. (2021). “Let Food Be Thy Medicine”: Gluten and Potential Role in Neurodegeneration. Cells, 10(4), 756. https://doi.org/10.3390/cells10040756
- Makhlouf, S., Messelmani, M., Zaouali, J., & Mrissa, R. (2018). Cognitive impairment in celiac disease and non-celiac gluten sensitivity: Review of literature on the main cognitive impairments, the imaging and the effect of gluten free diet. Acta Neurologica Belgica, 118(1), 21-27. https://doi.org/10.1007/s13760-017-0870-z
- Obrenovich, M. E. M. (2018a). Leaky Gut, Leaky Brain? Microorganisms, 6(4), Article 4. https://doi.org/10.3390/microorganisms6040107
- Obrenovich, M. E. M. (2018b). Leaky gut, leaky brain? Microorganisms, 6(4), 107. https://doi.org/10.3390/microorganisms6040107
- Obrenovich, M., Tabrez, S., Siddiqui, B., McCloskey, B., & Perry, G. (2020). The Microbiota-Gut-Brain Axis-Heart Shunt Part II: Prosaic Foods and the Brain-Heart Connection in Alzheimer Disease. Microorganisms, 8(4), Article 4. https://doi.org/10.3390/microorganisms8040493
- Philip, A., & White, N. D. (2022). Gluten, Inflammation, and Neurodegeneration. American Journal of Lifestyle Medicine, 16(1), 32-35. https://doi.org/10.1177/15598276211049345
- Sanz, Y. (2015). Microbiome and gluten. Annals of Nutrition and Metabolism, 67(Suppl. 2), 27-42. https://doi.org/10.1159/000440991
- Shabbir, U., Arshad, M. S., Sameen, A., & Oh, D.-H. (2021). Crosstalk between Gut and Brain in Alzheimer’s Disease: The Role of Gut Microbiota Modulation Strategies. Nutrients, 13(2), 690. https://doi.org/10.3390/nu13020690
- Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., Carlsson, C. M., Asthana, S., Zetterberg, H., Blennow, K., Bendlin, B. B., & Rey, F. E. (2017). Gut microbiome alterations in Alzheimer’s disease. Scientific Reports, 7(1), 13537. https://doi.org/10.1038/s41598-017-13601-y
- Yu, X. B., Uhde, M., Green, P. H., & Alaedini, A. (2018). Autoantibodies in the Extraintestinal Manifestations of Celiac Disease. Nutrients, 10(8), Article 8. https://doi.org/10.3390/nu10081123
- Zang, P., Gao, Y., Chen, P., Lv, C., & Zhao, G. (2022). Recent Advances in the Study of Wheat Protein and Other Food Components Affecting the Gluten Network and the Properties of Noodles. Foods, 11(23), Article 23. https://doi.org/10.3390/foods11233824
Image 1: from Wesual Click on Unsplash
Image 2: from (Shabbir et al., 2021)
Image 3: from Designua from Shutterstock



