Alzheimer gene triggers early collapse of the blood-brain barrier and predicts cognitive decline
Although scientists have known for a long time that apolipoprotein E4 is a leading genetic risk factor for Alzheimer’s disease, it has long been unknown how exactly it leads to memory loss. US researchers now believe they have an answer.
The gene Apolipoprotein E (ApoE) encodes an important lipid carrier protein in the brain, which is present in three different variants: ApoE2, ApoE3 and ApoE4. As with almost all genes, humans carry two copies of ApoE, which can be either the same or different variants. Compared to the most common ApoE3 variant, ApoE4 significantly increases the risk of Alzheimer’s: it is 4-fold higher in people with one copy of ApoE4 and up to 15-fold higher in people with two copies of this variant. Alzheimer’s patients who carry ApoE4 are more likely to develop symptoms of the disease earlier than carriers of other variants of ApoE (1). Read more about this in the chapter ‘Genetics‘.
This so-called late-onset Alzheimer’s disease is the most common form of dementia and is characterized by the extracellular accumulation of misfolded amyloid-β (Aβ) and intracellular aggregation of Tau proteins to form neurofibrillary tissues in the brain. It was long thought that the ApoE4 gene variant favours Aβ and Tau accumulation and thus contributes to the faster onset of Alzheimer’s disease. However, it is now clear that other damaging processes also play a role. For example, changes in the blood-brain barrier (BBB) have been shown to be early markers of this neurodegenerative disease. The degree of BBB dysfunction correlates directly with the extent of cognitive impairment, but which factors are responsible for its degradation was previously unknown. There is, currently, growing evidence that ApoE4, the leading genetic risk factor for Alzheimer’s disease, is related to BBB degradation (2).
But what exactly happens to the BBB and how is this in turn related to ApoE4 and the Aβ and Tau accumulation?
To fill this knowledge gap, the permeability of the BBB in healthy people and in patients with mild cognitive impairment was investigated and the results were correlated with their ApoE status. The researchers found that people who were cognitively healthy and carried either one or two copies of the ApoE4 variant had leaking BBBs in the hippocampus and parahippocampus – brain regions that play a role in memory formation and are involved in learning processes. This leaky BBB was more pronounced in ApoE4 carriers who suffered from a slight cognitive decline. In contrast, the BBB was intact in cognitively healthy ApoE3 carriers; But ApoE3 carriers who already showed cognitive impairment, presented also a leaky BBB.. Remarkably, these effects preceded all signs of tissue loss in affected brain regions, confirming that BBB dysfunction is a very early event in the onset of neurodegeneration, meaning that the integrity of this important barrier is lost before cognition weakens (3).
Follow-up studies have shown that BBB damage in ApoE4 carriers is associated with pericyte degeneration (2). Pericytes border on the endothelial cells are cells of the inner walls of the blood vessels, and thus protect the brain capillaries. They normally maintain the integrity of the BBB by preventing the breakdown of the connections between endothelial cells that make up the capillary walls (3). Pericyte destruction observed in ApoE4 carriers was independent of the accumulation of Aβ and Tau (2).
The mechanism of pericyte damage is now also known: these cells secrete the ApoE4 protein, which activates the protein cyclophilin A (CypA). This triggers a downstream signaling pathway that involves the activation of the inflammatory protein matrix metalloproteinase-9 (MMP9) in pericytes and possibly also in endothelial cells (4). This leads to an interruption of the connections between adjacent endothelial cells, which ultimately results in the disruption of the BBB in the hippocampus and parahippocampus. This mechanism is illustrated in the following figure (modified after Ref. 4):
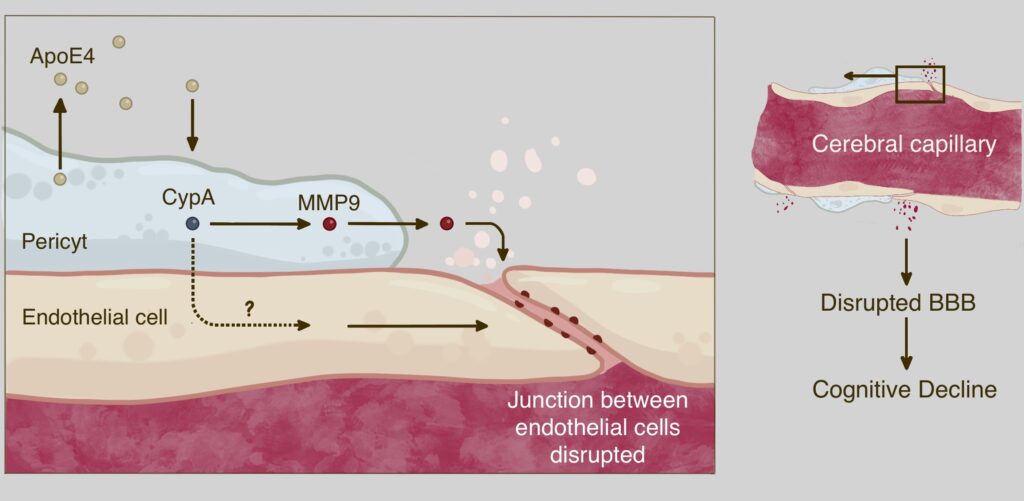
These observations thus shed new light on ApoE4, and contradicts the widely held idea that this gene variant contributes to Alzheimer’s disease simply by promoting Aβ and Tau accumulation.
Instead, the malfunctioning of the BBB seems to be responsible for the fact that ApoE4 carriers are susceptible to Alzheimer’s disease. This would also explain why ApoE4 carriers have a worse prognosis after a stroke or craniocerebral trauma than carriers of other ApoE variants (5).
Interestingly, these early mechanisms that trigger the cognitive impairment seem to be different among ApoE4 and ApoE3 carriers. It is possible that activation of the CypA pathway and damage to pericytes in ApoE3 carriers are not involved in cognitive impairment. However, it remains unclear, whether a leaking BBB caused by pericyte-independent factors such as endothelial cell damage by Aβ (6) contributes to cognitive impairment in ApoE3 carriers . Many other questions also remain unanswered, such as whether and how BBB degradation directly leads to cognitive impairment, or whether it is a cause or a consequence of the disease process. The role of the BBB in ApoE2 carriers, which was not investigated in the present study, is also still unknown. But the research results shed more light on understanding the role of the ApoE4 gene. It may be possible to diagnose people with the ApoE4 gene at an early stage by examining the brain vessels, and individualized therapy approaches could be taken early to effectively counteract premature cognitive decline.
Conclusion:
Damage to the pericytes that protectively surround the brain capillaries and seal the blood-brain barrier leads to a decline in cognition. In people with the gene variant ApoE4, this breakdown of the blood-brain barrier appears to be accelerated via an inflammatory pathway. This damage to the brain capillaries occurs at a very early stage, even before the tissue loss in the hippocampus occurs and cognition decreases. These new findings may offer the chance of early diagnosis in high-risk patients via the brain vessels, which is a promising approach in the fight against premature cognitive decline.Furthermore, they reinforce that the breakdown of the blood-brain barrier is the triggering mechanism in the pathophysiology of Alzheimer’s disease, being the accumulation of Aβ and Tau, secondary events.
References:
- Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C.-C. & Bu, G. (2019): Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nature Rev. Neurol. 15, 501–518
- Montagne, A. et al. (2020): ApoE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76
- Profaci, C. P., Munji, R. N., Pulido, R. S. & Daneman, R. (2020): The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 217, e20190062
- Ishii, M. & Iadecola, C. (2020): Lipid carrier breaks barrier in Alzheimer’s disease. Nature 581, 31-3
- Mahley, R. W., Weisgraber, K. H. & Huang, Y. (2006): Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc. Natl Acad. Sci. USA 103, 5644–5651
- Cortes-Canteli, M. & Iadecola (2020): Alzheimer’s Disease and Vascular Aging C. J. Am. Coll. Cardiol. 75, 942–951





